ANOMASS 500
BLADE LONCOMPHARMA-ANIMALS
Steroid Profile
Dosage
| Cycle Type | Dosage |
|---|---|
| Beginner | 400-800mg Every week |
| Intermediate | 800-1200mg Every week |
| Advanced | 1200-1600mg Every week |
BLADE Stacking
| Cycle Type | Dosage |
|---|---|
| Beginner | CLEN-40 40-80mcg ED T3-MAX 25-50mg ED STANOZOL-10 50-100mcg ED ANAVAR 50-100mg ED |
| Intermediate | CLEN-40 40-80mcg ED T3-MAX 25-50mg ED STANOZOL-10 50-100mcg ED ANAVAR 50-100mg ED WINSTROL-100 400-600mg EW hGH FRAG 500-1000mcg ED |
| Advanced | ANAVAR 50-100mg ED And/Or STANOZOL-10 50-100mg ED PROPIONE-T100 400-600mg EW CLEN-40 40-80mcg ED T3-MAX 25-50mg ED STANOZOL-10 50-100mcg ED ANAVAR 50-100mg ED WINSTROL-100 400-600mg EW hGH FRAG 500-1000mcg ED hGH 2-5iu ED |
Cycle Support**
| Cycle Type | Dosage |
|---|---|
| Beginner | Letrozole 1mg thrice EW Finasteride 2.5mg ED |
| Intermediate | Letrozole 1mg thrice EW Finasteride 2.5mg ED |
| Advanced | Letrozole 1mg thrice EW Finasteride 2.5mg ED |
PCT FOR BLADE CYCLE
| Cycle Type | Dosage |
|---|---|
| Beginner | Letrozole 1mg thrice EW HCG 1000-2000iu EW Clomid 50mg ED |
| Intermediate | Letrozole 1mg Thrice EW HCG 1000-2000iu EW Clomid 50mg ED |
| Advanced | Letrozole 1mg Thrice EW HCG 1000-2000iu EW Clomid 50mg ED |
Side Effects, Overdosage & Contraindications
Androgenic side effects such as oily skin, acne, seborrhoea, increased facial/body hair growth, scalp hair loss, and virilization may occur. Estrogenic side effects such as gynecomastia and fluid retention can also occur. STOP THE CYCLE AND SEEK EXPERT GUIDANCE IN CASE OF SEVERE SIDE EFFECTS. Disclaimer: Above mentioned information is for educational and references purposes only. Consult a registered healthcare professional proper course of medication.| In the process of continuous research and testing to make the most efficient and effective Products, Methylcobalamine ester is attached to the API in All 3rd Degree Oil-based injections. As per clinical trials done in association with the School of Medicine, Tui Tinh, Hanoi this inclusion, increases drug absorption by 25%. This also may cause the solution to look red or brownish in color. Please refer to clinical trial data in WHYLONCOMPHARMA > RESEARCH AND CLINICAL TRIALS |
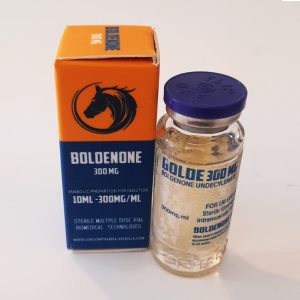
BOLDENONE 300 MG
Boldenone Undecylenate 300 mg/ml 10ml vial
Specifications:
Company: LONCOMPHARMA-ANIMALS Active Half-life (Days): 16 days Group: Anabolic Steroids Subgroup: Injection Dosage: 300 mg/ml Application (Men): 250-1500 mg a week Product pack: 10ml vial Content (active): Boldenone Undecylenate Oil-based: Yes Retains water: No Aromatization: NoBOLDY HDY LONCOMPHARMA ANIMALS
Dosage
| Cycle Type | Dosage |
|---|---|
| Beginner | 250-500mg Every week |
| Intermediate | 500-750mg Every week |
| Advanced | 750-1000mg Every week |
Stacking
| Cycle Type | Dosage | Duration |
|---|---|---|
| Beginner | PROPIONE-T100 200-300mg EW Or ENANTHA-T250 250mg EW Or CYPIONE-T250 250mg EW | 12-16 weeks |
| Intermediate | ANDROXY-50 50-100mg ED And/Or DIANABOL 50mg ED PROPIONE-T100 200-300mg EW Or ENANTHA-T250 250mg EW Or CYPIONA-T250 250mg EW DECA-D250 250-500mg EW | 12-16 weeks |
| Advanced | ANDROXY-50 50-100mg WD And/Or DIANABOL 50-100mg ED SUSTANON-T250 500-700mg EW DECA-D250 500-700 EW | 12-16 weeks |
Cycle Support**
| Cycle Type | Dosage |
|---|---|
| Beginner | Tamoxifen Citrate 10mg EOD |
| Intermediate | Tamoxifen Citrate 10-20mg ED |
| Advanced | Letrozole 1mg Thrice EW |
PCT CYCLE
| Cycle Type | Dosage | Duration |
|---|---|---|
| Beginner | Tamoxifen Citrate 10mg EOD Clomid 50mg EOD | 30 days |
| Intermediate | Tamoxifen Citrate 20mg ED HCG 1000-2000iu EW Clomid 50mg ED | 30 days |
| Advanced | Letrozole 1mg Twice a week HCG 1000-2000iu EW Clomid 50mg ED | 30 days |
Side Effects, Overdosage & Contraindications
Androgenic side effects such as oily skin, acne, seborrhoea, increased facial/body hair growth, scalp hair loss, and virilization may occur. Estrogenic side effects such as gynecomastia and fluid retention can also occur. STOP THE CYCLE AND SEEK EXPERT GUIDANCE IN CASE OF SEVERE SIDE EFFECTS. Disclaimer: Above mentioned information is for educational and references purposes only. Consult a registered healthcare professional for a proper course of medication.| In the process of continuous research and testing to make the most efficient and effective Products, Methylcobalamine ester is attached to the API in All 3rd Degree Oil-based injections. As per clinical trials done in association with the School of Medicine, Tui Tinh, Hanoi this inclusion, increases drug absorption by 25%. This also may cause the solution to look red or brownish in color. Please refer to clinical trial data in WHY 3RD DEGREE > RESEARCH AND CLINICAL TRIALS |
BULK-BOMBA LONCOMPHARMA-ANIMALS
Dosage
| Cycle Type | Dosage |
|---|---|
| Beginner | 500-1000mg EW |
| Intermediate | 1000-1500mg EW |
| Advanced | 1500-2000mg EW |
Stacking
| Cycle Type | Dosage |
|---|---|
| Beginner | ANDROXY-50 50-100mg ED And/or DIANABOL 50mg ED |
| Intermediate | ANDROXY-50 50-100mg ED And/or DIANABOL 50mg ED TRENBOL-A100 400-600mg EW |
| Advanced | ANDROXY-50 50-100mg ED And/or DIANABOL 50mg ED TRENBOL-A100 400-600mg EW hGH 2-5iu ED |
Cycle Support**
| Cycle Type | Dosage |
|---|---|
| Beginner | Tamoxifen Citrate 20mg EOD |
| Intermediate | Letrozole 1mg thrice EW |
| Advanced | Letrozole 1mg thrice EW |
PCT FOR BULK-BOMBA CYCLE
| Cycle Type | Dosage | Duration |
|---|---|---|
| Beginner | Letrozole 1mg Thrice EW HCG 1000-2000iu EW Clomid 50mg ED | 30 days |
| Intermediate | Letrozole 1mg Thrice EW HCG 1000-2000iu EW Clomid 50mg ED | 30 days |
| Advanced | Letrozole 1mg Thrice EW HCG 1000-2000iu EW Clomid 50mg ED | 30 days |
Side Effects, Overdosage & Contraindications
Androgenic side effects such as oily skin, acne, seborrhoea, increased facial/body hair growth, scalp hair loss, and virilization may occur. Estrogenic side effects such as gynecomastia and fluid retention can also occur. STOP THE CYCLE AND SEEK EXPERT GUIDANCE IN CASE OF SEVERE SIDE EFFECTS. Disclaimer: Above mentioned information is for educational and references purposes only. Consult a registered healthcare professional for a proper course of medication.| In the process of continuous research and testing to make the most efficient and effective Products, Methylcobalamine ester is attached to the API in All 3rd Degree Oil-based injections. As per clinical trials done in association with the School of Medicine, Tui Tinh, Hanoi this inclusion, increases drug absorption by 25%. This also may cause the solution to look red or brownish in color. Please refer to clinical trial data in WHY LONCOMPHARMA > RESEARCH AND CLINICAL TRIALS |
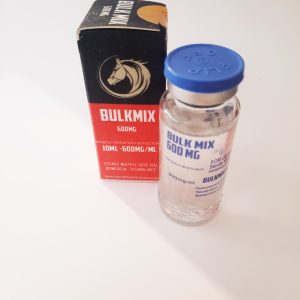
BULKMIX LONCOMPHARMA-ANIMALS
Description
Product Description: Testosterone Enanthate Injection is a pharmaceutical formulation containing testosterone enanthate, a synthetic derivative of the primary male hormone testosterone. It is indicated for the treatment of conditions associated with testosterone deficiency, such as hypogonadism and delayed puberty. This injection provides a convenient and effective way to administer testosterone replacement therapy for therapeutic purposes. Indications and Usage: Testosterone Enanthate Injection is indicated for testosterone replacement therapy in males with conditions associated with testosterone deficiency, including primary hypogonadism, secondary hypogonadism, and delayed puberty. It is used to restore normal testosterone levels and alleviate symptoms such as fatigue, decreased libido, and erectile dysfunction. Dosage and Administration: The dosage of Testosterone Enanthate Injection varies depending on the patient's age, weight, and the severity of testosterone deficiency. It is administered via intramuscular injection under the supervision of a healthcare professional. The injection site should be rotated to minimize the risk of local tissue irritation. The dosage regimen should be individualized based on the patient's clinical response and any underlying medical conditions. Dosage Forms and Strengths: Testosterone Enanthate Injection is available as a sterile solution in single-dose vials. Each mL of solution contains testosterone enanthate equivalent to 200 mg or 250 mg of testosterone. Contraindications: Testosterone Enanthate Injection is contraindicated in patients with known or suspected prostate or breast cancer, male breast carcinoma, or hypersensitivity to testosterone enanthate or any of the excipients. It should not be used in females, especially during pregnancy or lactation, due to the risk of virilization in the fetus or newborn. Warnings and Precautions: Administration of Testosterone Enanthate Injection requires careful monitoring of the patient's clinical response and any signs of adverse effects. It should be used with caution in patients with a history of cardiovascular disease, hepatic impairment, or renal dysfunction, as testosterone replacement therapy may exacerbate these conditions. Long-term use may be associated with an increased risk of prostate enlargement or prostate cancer. Adverse Reactions/Side Effects: Common adverse reactions associated with Testosterone Enanthate Injection include injection site reactions, such as pain, swelling, or redness. Other adverse effects may include acne, fluid retention, gynecomastia, and changes in mood or libido. Rare but serious adverse reactions may include cardiovascular events, such as myocardial infarction or stroke, or thromboembolic events, such as deep vein thrombosis or pulmonary embolism. Drug Interactions: Testosterone Enanthate Injection may interact with other medications that affect hormonal balance or liver metabolism, such as corticosteroids, anticoagulants, or insulin. Concurrent use with drugs that induce hepatic enzymes, such as barbiturates or rifampin, may increase the metabolism of testosterone, leading to decreased efficacy. Close monitoring is recommended when administering testosterone with other medications affecting the cardiovascular system or coagulation cascade. Use In Specific Populations Description: The safety and efficacy of Testosterone Enanthate Injection in pediatric patients, geriatric patients, and pregnant or lactating women have not been established. Use with caution in these populations, and only if the potential benefits outweigh the risks. Close monitoring is recommended in patients with renal or hepatic impairment. How Supplied/Storage and Handling: Testosterone Enanthate Injection is supplied as a sterile solution in single-dose vials. It should be stored at controlled room temperature between 20°C to 25°C (68°F to 77°F), protected from light and moisture. Do not freeze. Discard any unused portion of the solution.Composition:
Each 1 mL ampoule/vial contains:
-
Nandrolone Decanoate … 175 mg
(in a sterile oily vehicle for intramuscular administration)
Therapeutic Category:
Anabolic Steroid
Uses of Nandrolone Decanoate
This medication is used for mainly three major reasons: Postmenopausal Osteoporosis Nandrolone Decanoate Injection is used to treat and manage the symptoms of Osteoporosis in postmenopausal women. It is used to prevent bone fractures and increase bone density. Anemia with Renal Insufficiency Nandrolone Decanoate is used for the management of anemia in patients who have impaired kidney function. Debilitating Illness This medicine is also used for a speedy recovery from an illness that seriously affects the strength of the patient.Side Effects
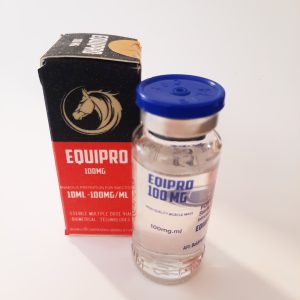
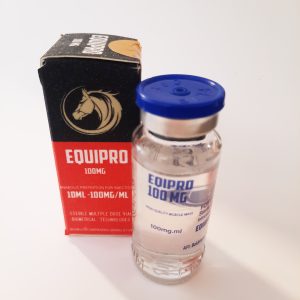
API: Boldenone Undecylenate -200 mg/ml
Usage: BOLD-E250 commonly known as Equipoise or boldenone undecylenate, injectable steroids with strong anabolic and lower androgenic properties. The undecylenate ester extends the half life making it effective with less frequent injections. Boldenone undecylenate is also commonly known as a drug capable of increasing red blood cell production, with muscle building effects similar to deca.CARRIER LOCOM PHARMA- ANIMA
CUT RIP LONCOMPHARMA-ANIMALS
About Product
CUT PRO MAX previously know as “I.P. BLEND 6” is presented in a 10-milliliter multi-dose vial and reportedly contains the following four steroid compounds: 125 milligrams of testosterone propionate, 125 milligrams of testosterone phenylpropionate, 125 milligrams of trenbolone acetate and 125 milligrams of drostanolone propionate per milliliter. It is a unique combination developed by LONCOM PHARMA for contest preparation.EQUIPRO LONCOMPHARMA-ANIMALS
Dosage
| Dose | As per Physician |
| Duration | NA |
| In the process of continuous research and testing to make the most efficient and effective Products, Methylcobalamine ester is attached to the API in All LONCOM PHARMA Oil-based injections. As per clinical trials done in association with the School of Medicine, Tui Tinh, Hanoi this inclusion, increases drug absorption by 25%. This also may cause the solution to look red or brownish in color. Please refer to clinical trial data in WHY 3RD DEGREE > RESEARCH AND CLINICAL TRIALS |